Psoriasis
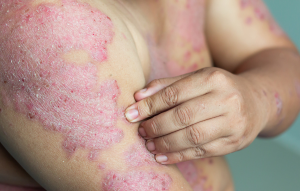
CHRONIC PLAQUE PSORIASIS RESEARCH TRIAL (24 WEEKS)
Adults aged 18 years or older with a clinical diagnosis of plaque psoriasis of at least 6 months duration, may qualify for this research trial and receive investigational topical treatment (active study drug) or placebo.
Individuals must have the disease affecting 3%-15% of the body surface area (BSA) (excluding head) to be eligible for topical treatment. Adults must not have other types of psoriasis dominant other than plaque psoriasis. During the study period, participants must also agree not to have prolonged sun exposure. Eligible participants will have regular visits with our experienced research team.
There is no cost to participate, and participants will be reimbursed for study-related expenses.
Individuals will be asked to visit the study centre about 8 times over a period of about 24 weeks.
For more information, please contact:
Principal Investigator
A/Prof Peter Foley
Sub Investigators
Dr Nisal Punchihewa
t: 9623 9464
e: [email protected]
Study Coordinator
Natasha Harrison
t: 9623 9435
e: [email protected]
PLAQUE PSORIASIS RESEARCH STUDY (61 WEEKS)
Adults who are 18 years of age or older who have a diagnosis of chronic plaque psoriasis for at least 6 months, may qualify for this clinical research study and receive the investigational treatment (oral study drug), comparator (an approved oral treatment for moderate to severe plaque psoriasis), or placebo (a substance that looks similar to the investigational treatment but does not contain any active ingredients).
Eligible participants must have moderate to severe plaque psoriasis, with psoriasis covering at least 10% of their total body surface area (BSA), and be a candidate for phototherapy or systemic therapy.
Participants will have regular visits with an experienced research team. There is no cost to participate, and participants will be reimbursed for study-related expenses.
Individuals will be asked to visit the study centre about 18 times over the course of about a year.
For more information, please contact:
Principal Investigator
A/Prof Peter Foley
Sub Investigators
Dr Nisal Punchihewa
t: 9623 9464
e: [email protected]
Study Coordinator
Bailey Elward
t: 9623 9425
e: [email protected]

