HIDRADENITIS SUPPURATIVA RESEARCH STUDY
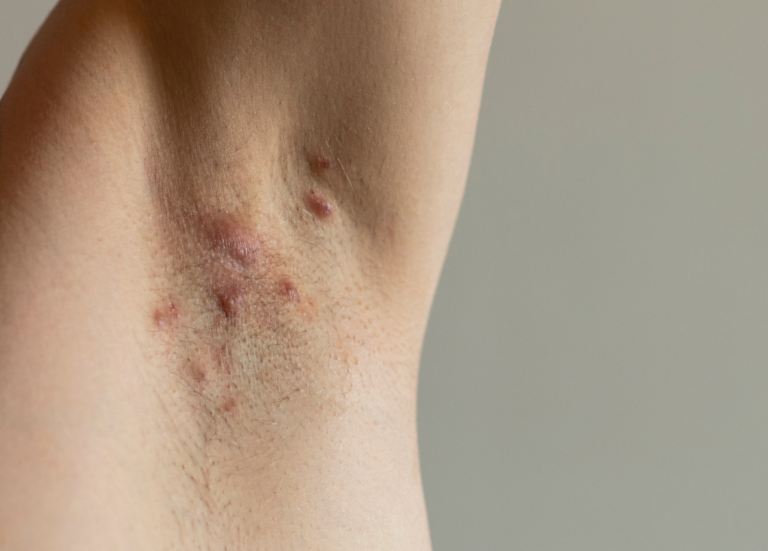
RESEARCH STUDY FOR HIDRADENITIS SUPPURATIVA (62 WEEKS)
Adults who are 18 years of age or older with a diagnosis of moderate to severe hidradenitis
suppurativa (HS) for at least 3 months, may qualify for this clinical research study and receive
investigational oral medication (active study drug) or placebo via tablets for the initial 12 weeks. After
that part of the study, all participants who continue in the study will receive active study drug for 42 weeks.
Eligible participants must have at least 5 active HS lesions (such as bumps, boils, and/or
abscesses); these HS lesions need to be present in at least 2 different body areas (for example, left
armpit and right armpit). Eligible participants must have been prescribed a previous HS treatment
(taken by mouth, or taken via injection) that did not improve their symptoms.
Participants will have regular study-related visits with an experienced research team. There is no
cost to participate to the participant or their insurer, and qualified participants will be reimbursed for their time and travel.
Individuals will be asked to visit the study centre about 15 times over a period of about 62 weeks.
For further information on the study, please follow the link:
For more information, please contact:
Principal Investigator
A/Prof Peter Foley
Sub Investigators
Dr Nisal Punchihewa
t: 9623 9464
e: [email protected]
Study Coordinator
Irina Danilovich
t: 9623 9448
e: [email protected]
RESEARCH STUDY FOR HIDRADENITIS SUPPURATIVA (28 MONTHS)
Adults with a diagnosis of hidradenitis suppurativa (HS) for at least 6 months may qualify for this clinical research trial and receive investigational oral treatment (active study drug) or placebo(inactive; contains no medicine).
Eligible participants must have HS lesions present in at least 2 distinct anatomic areas, have a total abscess and inflammatory nodule (AN) count of 5 or more, and a draining tunnel (fistula) count of 20 or less. In addition, individuals must have a documented history of previous use of 1 or more TNF inhibitors for HS for at least 12 weeks and/or 1 approved non-anti-TNF biologic therapy for HS for atleast 16 weeks characterized by inadequate response or intolerance.
Participants will have regular visits with an experienced research team. There is no cost to participate, and participants will be reimbursed for study-related expenses.
Individuals will be asked to visit the study centre about 19 times over a period of about 28 months.
For further information on the study, please follow the link:
Principal Investigator
A/Prof Peter Foley
Sub Investigators
Dr Nisal Punchihewa
t: 9623 9464
e: [email protected]
Study Coordinator
Bailey Elward
t: 9623 9425
e: [email protected]

