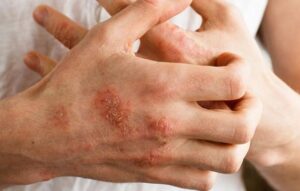
Atopic Dermatitis
MODERATE ATOPIC DERMATITIS RESEARCH TRIAL (34 WEEKS)
Adults aged over 18 years who have a diagnosis of atopic dermatitis (AD), with AD duration of at least 2 years, may qualify for this clinical research trial and receive investigational topical treatment (active study drug), or placebo.
Eligible participants must have moderate atopic dermatitis, with AD involvement (excluding the scalp) of at least 10% and up to 20% body surface area (BSA), along with documented recent history (within 12 months before the screening visit) of inadequate response, intolerance, or contraindication to topical corticosteroids and topical calcineurin inhibitors.
Participants will have regular visits with an experienced research team. There is no cost to participate, and participants will be reimbursed for study-related expenses.
Individuals will be asked to visit the study centre about 10 times over a period of about 34 weeks.
For more detailed information about this clinical trial, please visit:
Principal Investigator
A/Prof Peter Foley
Sub Investigator
Dr Nisal Punchihewa
t: 9623 9464
e: [email protected]
Study Coordinator
Natasha Harrison
t: 9623 9435
e: [email protected]
You can register your interest for this trial via the button below.

